
Q-Cell® Technology
OUR UNIQUE APPROACH
Q Therapeutics is developing innovative cell therapies for debilitating CNS diseases by boosting the body’s natural support and repair mechanisms to restore neuron function and health.
Regardless of the underlying cause, mechanism or pathways involved, what most CNS injuries and diseases have in common is the degeneration, and even death, of neurons. Part of the body’s natural response to CNS injury and disease is to increase the number of glial cells in order to support and promote normal neuron health. However, the body’s ability to produce glial cells is limited, and the resulting increase may not be sufficient to repair the damage. Furthermore, in some neurodegenerative diseases, the body's own glial cells are diseased.
Although the neuron is the brain cell that people are most familiar with, the brain is also made up of approximately 85 billion glial cells (glia). Glia are the unsung heroes that provide essential support and repair functions and even outnumber neurons in many regions of the brain. Without healthy glial cells, neurons degenerate and may even die.
Q Therapeutics is uniquely focused on a glial cell therapeutic approach to treating neurodegenerative diseases and damage to the brain
Q-Cells® – our first cellular therapeutic candidate – are human glial progenitor cells that give rise to two types of specialized glial cells, astrocytes and oligodendrocytes.
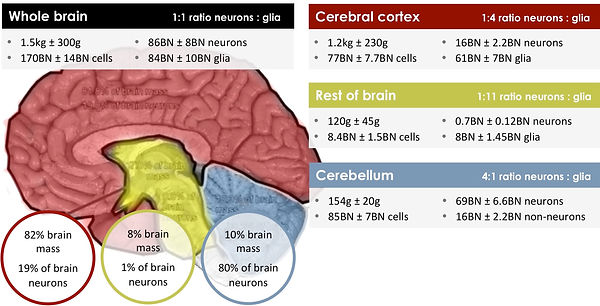
UNDERSTANDING THE ROLE OF GLIA
The most common types of glial cells in the brain are:
-
Astrocytes – Specialized cells that protect neurons by producing growth factors, removing toxins, protecting against damage, modulating the body's immune response and providing structural support.
-
Oligodendrocytes – Specialized cells that insulate neurons by producing myelin, the fatty insulation required for normal nerve transmission.

Unlike neurons, glial progenitor cells have the power to:
-
Replicate and Multiply following injection into the brain or spine
-
Migrate, or move, to sites of injury or disease in the brain or spine
-
Differentiate into two specialized cell types – astrocytes and oligodendrocytes – that are essential for normal function of nerve cells in the brain and spine
-
Augment the body’s natural protective response to nerve cell injury
In addition to producing the extracellular support matrix that holds neurons in place, glial cells also:
-
Provide neurons with nutrients and growth factors
-
Remove toxins and cellular debris
-
Protect neurons from damage and modulate the body's immune response
-
Produce myelin, a fatty substance that insulates the electrical impulse-transmitting axons of neurons
A growing body of research on the significance of glia is driving the paradigm shift that informs and supports Q Therapeutics’ cellular therapeutic approach to treating diseases of the central nervous system (CNS, or the brain and spinal cord).
WHAT MAKES Q-CELLS® SPECIAL
Q-Cells® are the first and only human glial-restricted progenitor (GRP) cell therapeutic. Q Therapeutics is the only company developing this specialized cell type, which we believe to have the greatest potential for long-lasting CNS benefits.
Glial progenitor cells are early descendants of neural stem cells that can produce two types of glial cells that are essential for supporting, maintaining or even restoring neuron health.
Although glial progenitor cells are present in the adult brain (including the spinal cord), they are limited in number.
Q-Cells® are purified human glial progenitor cells that have been isolated and cultured from brain tissue at the precise stage of development when their ability to multiply, migrate and differentiate into glial cells is most potent.
-
Not harvested from the patient – Q-Cells® are an allogeneic cell therapy delivered to the site as an off-the-shelf pharmaceutical product that is ready to be injected for therapeutic use.
-
No genetic manipulation – Q-Cells® are isolated and purified from brain tissue, so they are natural CNS cells in their natural state performing their natural function.
-
Small-volume injection – Each vial of Q-Cells® contains millions of human glial progenitor cells, with the capacity to multiply and differentiate into millions of astrocytes and oligodendrocytes.
-
Long-lived in the CNS – Q-Cells® survive in the CNS longer than mesenchymal stem cells (MSCs), with the potential to provide long-lasting effects.
Based on numerous, promising laboratory and animal studies, we are actively preparing for a clinical trial of Q-Cells® in human patients with transverse myelitis and amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease). In 2017, we received FDA clearance to proceed with a 9 patient, dose escalating trial in transverse myelitis patients that complements our IND clearance for a 30 patient, dose escalating trial in ALS.
Q-Cells® have been shown to successfully differentiate into:
-
Astrocytes – Specialized cells that perform a variety of neuroprotective functions
-
Oligodendrocytes – Specialized cells that produce the myelin needed for normal transmission of nerve signals in the brain and spinal cord
Q-Cells® have already been granted fast-track and orphan disease designation by the U.S. Food and Drug Administration (FDA) for ALS. In addition, Q-Cells have been granted orphan disease designation for Transverse Myelitis. Going forward, we will be pursuing similar designations in both Europe and Japan.
Existing safety and manufacturing data support multiple CNS indications for broader market penetration.
Q Therapeutics’ patent estate consists of 21 issued patents and additional U.S. and international patents pending, providing a strong intellectual property position for Q-Cells®, as well as other neural lineage cells. Q Therapeutics has exclusive worldwide rights to our Q-Cells® product through an agreement with the University of Utah Research Foundation and through owned, internally-developed intellectual property.
Our patent portfolio encompasses five families of neural lineage progenitor or stem cell technologies. Currently, our patent estate encompasses composition of matter, methods of production and methods of use for multiple cell types of the central nervous system (including Q-Cells®), as well as some cell types of the peripheral nervous system.
PRE-CLINICAL EVIDENCE OF SAFETY
Q-Cells® have already been studied extensively in the laboratory and in rodent models of various CNS diseases, including:
-
Myelination deficiency, as is found in multiple sclerosis
-
Amyotrophic lateral sclerosis (ALS, a degenerative disease of the motor neurons)
-
Spinal cord injury
Rodent models are excellent models for many human CNS diseases because CNS mechanisms are highly conserved (have remained largely unchanged by evolution) between rodents and humans. The research results in these highly conserved models of human disease are promising for our planned phase 1/2a clinical trials in transverse myelitis and ALS.
Pre-clinical studies provide strong evidence that Q-Cells® have the potential to restore neuron function and improve survival in demyelinating and neurodegenerative diseases. Pre-clinical studies have shown:
-
No observed toxicity in more than 360 treated animals in six independent laboratories
-
Q-Cells® differentiate only into astrocytes and oligodendrocytes
-
Q-Cells® are capable of migrating to and targeting at-risk motor neurons in animal models of transverse myelitis and ALS
-
Implantation of Q-Cells® in animal models of transverse myelitis and ALS enhances motor function and survival*
-
Q-Cells® can mature into oligodendrocytes that are capable of producing normal myelin
-
A single implantation of Q-Cells® results in widespread myelin production, with associated improvement in survival
Q-Cells® do not differentiate into neurons, eliminating the risk of uncontrolled new neuron formation or aberrant neuronal connections that could lead to seizures or other nerve conduction problems. In addition, Q-Cells® can be injected directly into the brain or spinal cord at the site of injury or disease, avoiding many of the off-target risks associated with oral medicines or medicines delivered through the bloodstream.
PRE-CLINICAL EVIDENCE OF EFFICACY
Pre-clinical studies provide strong evidence that Q-Cells® have the potential to restore neuron function and improve survival in neurodegenerative diseases.
In Diseases Involving Neuron Damage
Amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) is a neurodegenerative disease that affects motor neurons, the nerve cells in the brain and spinal cord that control muscle movement. The progressive degeneration of motor neurons caused by ALS eventually leads to motor neuron demise. The most common cause of death among ALS patients is respiratory failure, which occurs when nerve damage eventually affects the muscles that control breathing.
In animal models of ALS, transplantation of Q-Cells® has shown that Q-Cells® localize to motor neurons in the spinal cord, positioned to rescue those neurons that are at risk.

Studies have also shown enhanced survival and motor function in ALS rats treated with the rat homolog of Q-Cells®.
In Diseases Involving Myelin Deficiency (Glial Cell Damage)
Demyelinating diseases, such as multiple sclerosis and transverse myelitis, occur when myelin-producing oligodendrocytes are damaged or destroyed. In animal models of myelin deficiency, transplantation of Q-Cells® has been shown to give rise to oligodendrocytes that are able to produce functional, widespread myelination and associated survival benefit.

Q-Cells® Migrate
Unlike neurons, Q-Cells® can migrate, enabling them to travel to and target sites of CNS injury or disease.
Based on numerous, promising laboratory and animal studies, we are actively preparing for a clinical trial of Q-Cells® in human patients with transverse myelitis and amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease). We have received FDA clearance to proceed and expect to begin transverse myelitis trials in 2019.
